Hand tremor reduction for patients with Essential tremor
The results from the research shows that VILIM ball device can effectively reduce hand tremors. There are many ways to prove such an effect. One of the most popular ones is an Archimedes spiral drawing. Smooth spiral drawing means that a person does not have any hand tremor symptoms. Wobbly, uneven and noisy spiral drawing can mean that the person do have hand tremor in action state. Such an approach was used in one of our studies. The results were positive – VILIM ball device makes spiral drawings smoother. In other words, the device reduces hand shakiness. Image below illustrates findings from this particular study.

Clinical investigations
Currently there are at least 2 published clinical studies regarding the VILIM ball device. Efficacy study No. 1 was a prospective, single-center, randomized, controlled study designed to evaluate device efficacy. 17 subjects with diagnosed Essential tremor were randomly selected. VILIM ball therapy was performed for each patient on dominant hand. Collected data was filtered to evaluate the range of 4-12 Hz which is hand tremor frequency with diagnosed essential tremor. The study concluded, that the device reduced tremor power from a mean of 0.106 to the mean of 0.042. In other words, the tremor was reduced on average by ~60%. Improvement was noticed for 13 patients according to tremor power measurements. Following publication, the study was expanded to gather more evidence required for device certification in the European Union. The research was repeated with an additional group of patients using a sham device. The findings concluded that the sham device had no effect on tremor.

*Abramavičius, Silvijus; Venslauskas, Mantas; Vaitkus, Antanas; Gudžiūnas, Vaidotas; Laucius, Ovidijus; Stankevičius, Edgaras. Local vibrational therapy for essential tremor reduction: a clinical study // Medicina. Basel : MDPI AG. ISSN 1010-660X. eISSN 1648-9144. 2020, vol. 56, iss. 10, art. no. 552, p. 1-9. DOI: 10.3390/medicina56100552.
The second VILIM ball efficacy study was a cross-sectional study performed on patients with Essential and Parkinsonian tremors. In total 50 patients with the mean age of 66.9 were included (30 in the essential tremor and 20 Parkinsonian tremor). The primary efficacy outcome was the Patient-Reported Outcome based on a non-validated patient telephone questionnaire. The secondary outcome was the occurrence of adverse events. 46 patients reported improvement in tremor symptoms and function. 4 patients (2 PD and 2 ET) reported lack of effect in terms of symptoms and function. The patients used the VILIM ball for 7.63 months. 38 patients were able to report the duration of improved function which was 90.79 minutes. 4 more patients refused to participate in the study because of no improvement in tremor during the two-week initial testing phase. No adverse events were reported by the patients in this study.
These results proves that the VILIM ball therapy is effective and can reduce hand tremor symptoms. The further researches will be dedicated for the optimization of the device effectiveness.

Post-market studies
Customer surveys, documented in efficacy study no. 2, were continued after the device was introduced to the market. A voluntary questionnaire is periodically sent to all customers who bought the device and kept it for 2 weeks to 2 years. This activity aims to gather real-world evidence about how the device performs when is presented to the customer. By processing survey results we get an idea of the device efficacy level and how it changes during short to long periods.
In the years 2023 and 2024, we additionally surveyed 143 customers with a mean age of 67 years and an average VILIM ball usage time of 1.88 months. Most of the respondents were patients with Essential tremor (ET), however, 16% of customers were patients with Parkinson’s disease (PD) and 2.8% were diagnosed with other diseases. According to aggregated data, improvement was noticed by 70.4% of patients (74.4% PD and 69.2% ET). The average improvement time was 74 minutes (± 15 min.).
Even though our methods are constantly improving, it should be noted that self-reporting voluntary surveys in the medical field have several limitations, including:
- Selection Bias: Individuals who choose to participate may differ systematically from those who do not, potentially skewing the results.
- Recall Bias: Participants may not accurately remember past events or experiences, leading to inaccurate data.
- Social Desirability Bias: Respondents may provide answers they believe are more socially acceptable or favorable rather than their true thoughts or behaviors.

Studies on Parkinsonian tremors
High off-label use detected in the post-market surveillance reflects the need to investigate the device’s effect on other tremors, especially Parkinsonian. Communication about the intended use of labeling, marketing, or informational channels is sufficient. However, during non-formal communication with customers, it was noted that customers who use device off-label are typically aware that the device is meant solely for Essential Tremor. However, available solutions, mainly for for Parkinson’s disease, are not effective. Patients tend to seek help on other fronts. That includes VILIM ball. Various clinical studies regarding the VILIM ball therapy effect on Parkinsonian tremors are on-the-way.
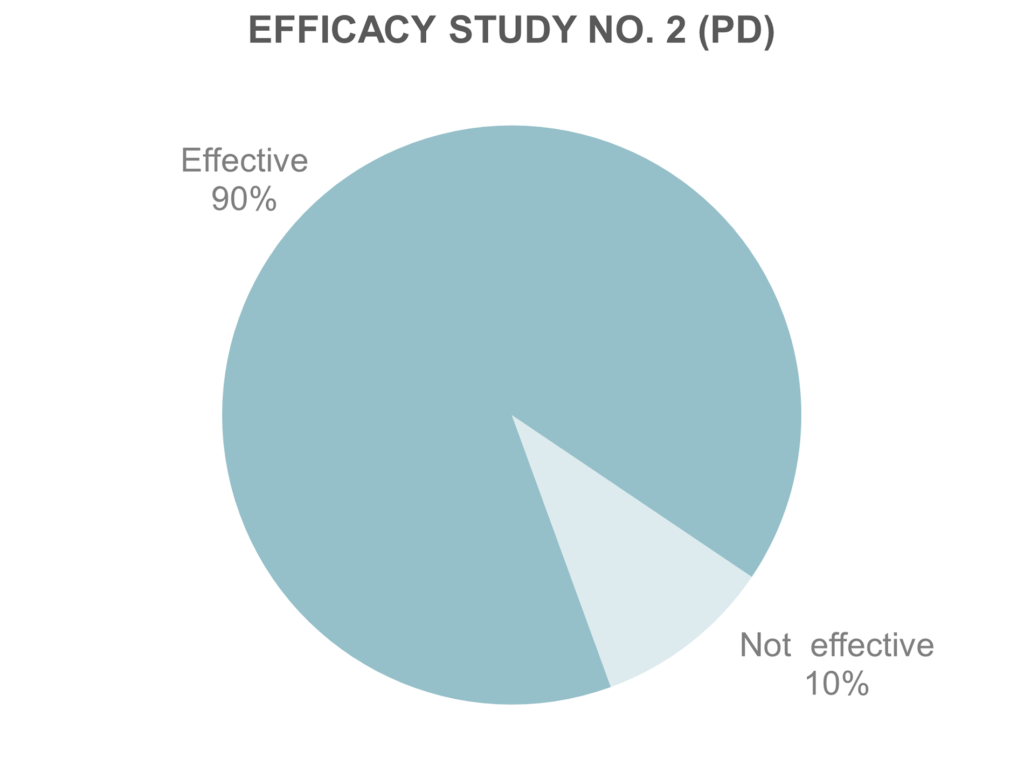
Efficacy study no. 2 included 20 patients with Parkinson’s Disease. 18 of them concluded reduction in tremor symptoms after therapy with the VILIM ball. Currently Dr. Dieter Volc, Specialist in Neurology and Psychiatry, is performing an internal study focused on VILIM ball therapeutic effect on Parkinsonian tremor patients. Even thought the conclusions are not yet public, latest recorded data concluded tremor reduction for 19 of 25 PD patients.

Currently, the VILIM ball device holds certification solely for Essential Tremor (ET) patients. Nevertheless, the available information strongly indicates that Parkinson’s Disease (PD) patients can also experience benefits from personalized mechanical vibration therapy. Consequently, Team VILIMED aims to pursue approval for extending the use of VILIM ball to PD patients in the near future.
Other studies
According to other similar study, the vibrational therapy could reduce hand tremor and stiffness for Parkinson’s disease by 25% and 24%, respectively. (Haas, 2006). Also, King et al. (2009) have found that vibrational therapy could significantly decrease rigidity, and tremor. Results of their initial investigation provide support for vibration therapy as a non-pharmacological treatment alternative.
A group of researchers performed study on combination of the whole body vibration therapy and exercises. It was observed that this kind of therapy gives short term benefit for human motor system, posture and daily activities (Edmonston , 2016).
Heiko (2009) and others have found that the whole body vibration therapy significantly reduces hand tremor and stiffness as well as step length and walking speed.
Certification
Vilimed has implemented the EN ISO 13485 quality management system standard for the medical device industry and was granted the CE0197 Class 2a mark for the VILIM ball device. The quality certificates were granted by the notified body TÜV Rheinland.




